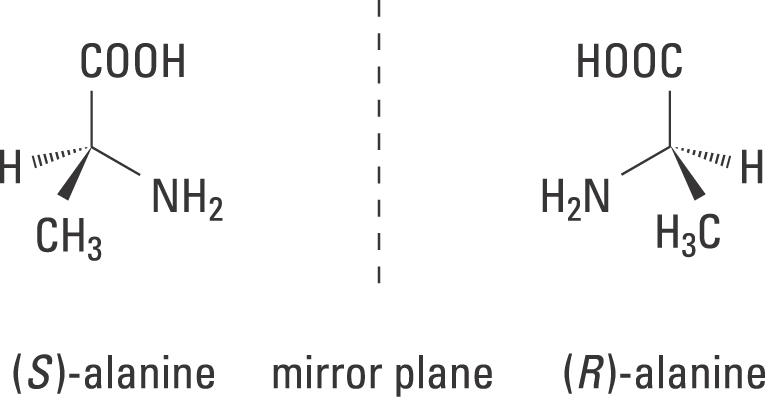
Let’s apply our chirality discussion to real molecules. Consider 2-butanol, drawn in two dimensions below. Carbon #2 is a chiral center: it is sp 3-hybridized and tetrahedral (even though it is not drawn that way above), and the four substituents attached to is are different: a hydrogen (H) , a methyl (-CH 3) group, an ethyl (-CH 2 CH 3) group, and a hydroxyl (OH) group.
Stereoisomers
One of the most interesting types of isomer is mirror-image stereoisomers, a non-superimposable set of two molecules that are mirror images of one another. The existence of these molecules is determined by concept known as chirality. The word “chiral” was derived from the Greek word for hand, because our hands are good example of chirality

Source Image: researchgate.net
Download Image
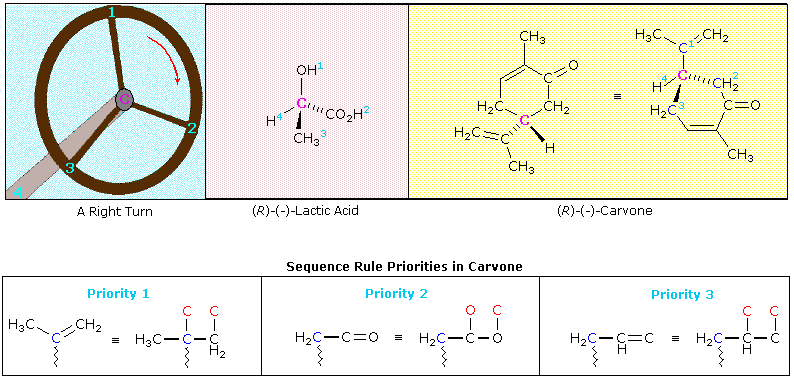
Rules for assigning an R/S designation to a chiral center. 1: Assign priorities to the four substituents, with #1 being the highest priority and #4 the lowest. Priorities are based on the atomic number. 2: Trace a circle from #1 to #2 to #3. 3: Determine the orientation of the #4 priority group.
Source Image: quora.com
Download Image
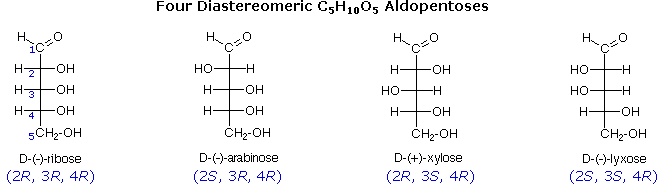
Stereoisomers Jul 20, 2022c) List all of the stereoisomers that are diastereomers, but not epimers, of SRS. The epimer term is useful because in biochemical pathways, compounds with multiple chiral centers are isomerized at one specific center by enzymes known as epimerases. Two examples of epimerase-catalyzed reactions are below.
Source Image: chem.libretexts.org
Download Image
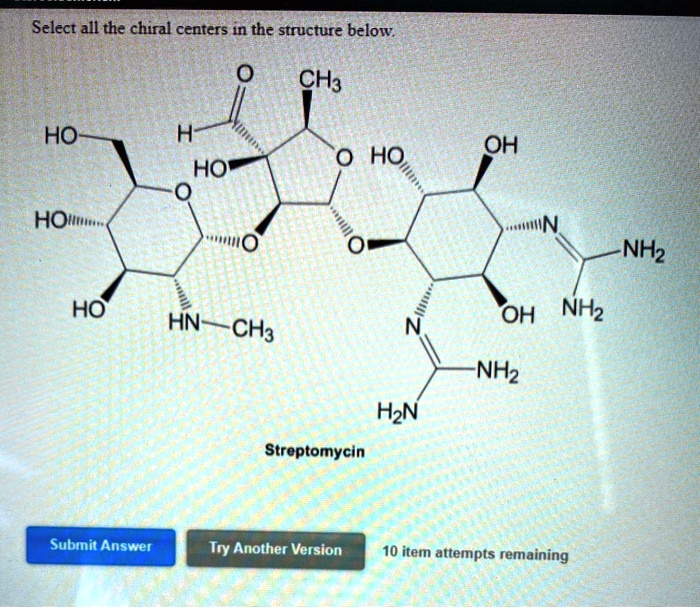
Select All The Chiral Centers In The Structure Below.
Jul 20, 2022c) List all of the stereoisomers that are diastereomers, but not epimers, of SRS. The epimer term is useful because in biochemical pathways, compounds with multiple chiral centers are isomerized at one specific center by enzymes known as epimerases. Two examples of epimerase-catalyzed reactions are below. A chiral centre is an atom that has four different groups bonded to it in such a manner that it has a nonsuperimposable mirror image. The term “chiral centre” has been replaced by the term chirality centre. In the molecule below, the carbon atom is a chirality centre. It has four different groups attached, and the two structures are
5.1: Chiral Molecules – Chemistry LibreTexts
Step 1 1 of 3 Our task here is to determine all the chiral centers in the structure of Streptomycin given below. Step 2 2 of 3 For the molecule to be chiral it must possess at least one chiral center (or stereocenter). The chiral center is a carbon atom that bears four different groups. How many chiral centers are present in this molecule?

Source Image: toppr.com
Download Image
SOLVED: Select all the chiral centers in the structure below: CH3 HO H HO” OH HO HOI NH2 HO OH NH2 HN CH3 NH2 H2N Streptomycin Submit Answer Try Another Version 10 Step 1 1 of 3 Our task here is to determine all the chiral centers in the structure of Streptomycin given below. Step 2 2 of 3 For the molecule to be chiral it must possess at least one chiral center (or stereocenter). The chiral center is a carbon atom that bears four different groups.
Source Image: numerade.com
Download Image
Stereoisomers Let’s apply our chirality discussion to real molecules. Consider 2-butanol, drawn in two dimensions below. Carbon #2 is a chiral center: it is sp 3-hybridized and tetrahedral (even though it is not drawn that way above), and the four substituents attached to is are different: a hydrogen (H) , a methyl (-CH 3) group, an ethyl (-CH 2 CH 3) group, and a hydroxyl (OH) group.
Source Image: www2.chemistry.msu.edu
Download Image
Stereoisomers Rules for assigning an R/S designation to a chiral center. 1: Assign priorities to the four substituents, with #1 being the highest priority and #4 the lowest. Priorities are based on the atomic number. 2: Trace a circle from #1 to #2 to #3. 3: Determine the orientation of the #4 priority group.
Source Image: www2.chemistry.msu.edu
Download Image
Finding R and S for Chiral Centers – Organic Chemistry | Socratic The rule of thumb is: chiral carbon centers are carbon atoms that are attached to four different substituents, that are placed at the corners of a tetrahedron. Chiral carbon atoms are also referred to as ‘stereogenic carbons’ or ‘asymmetrical carbon atoms’.
Source Image: socratic.org
Download Image
3.7: Compounds with multiple chiral centers – Chemistry LibreTexts Jul 20, 2022c) List all of the stereoisomers that are diastereomers, but not epimers, of SRS. The epimer term is useful because in biochemical pathways, compounds with multiple chiral centers are isomerized at one specific center by enzymes known as epimerases. Two examples of epimerase-catalyzed reactions are below.
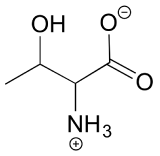
Source Image: chem.libretexts.org
Download Image
In the image map below, select all of the chiral centers in the molecule. | Homework.Study.com A chiral centre is an atom that has four different groups bonded to it in such a manner that it has a nonsuperimposable mirror image. The term “chiral centre” has been replaced by the term chirality centre. In the molecule below, the carbon atom is a chirality centre. It has four different groups attached, and the two structures are

Source Image: homework.study.com
Download Image
SOLVED: Select all the chiral centers in the structure below: CH3 HO H HO” OH HO HOI NH2 HO OH NH2 HN CH3 NH2 H2N Streptomycin Submit Answer Try Another Version 10
In the image map below, select all of the chiral centers in the molecule. | Homework.Study.com One of the most interesting types of isomer is mirror-image stereoisomers, a non-superimposable set of two molecules that are mirror images of one another. The existence of these molecules is determined by concept known as chirality. The word “chiral” was derived from the Greek word for hand, because our hands are good example of chirality
Stereoisomers 3.7: Compounds with multiple chiral centers – Chemistry LibreTexts The rule of thumb is: chiral carbon centers are carbon atoms that are attached to four different substituents, that are placed at the corners of a tetrahedron. Chiral carbon atoms are also referred to as ‘stereogenic carbons’ or ‘asymmetrical carbon atoms’.